Galvanic Cells
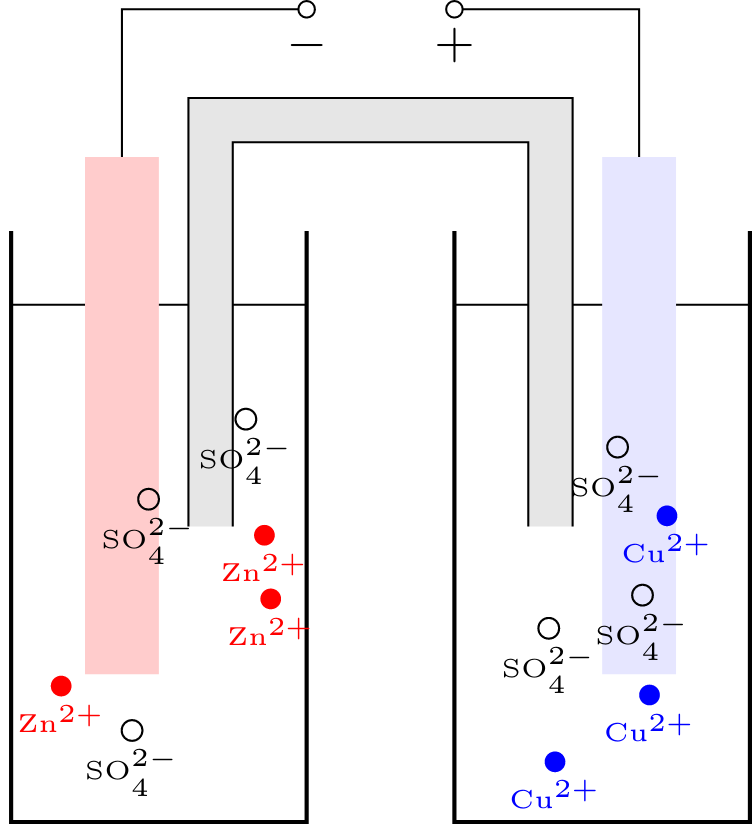
If you, for instance, dissolve zinc sulfate in water, you get zinc ions Zn${}^{2 }$ with a double positive charge and sulfate ions SO${}^{2-}$ with a double negative charge. If you now put a rod made of pure zinc into the liquid, zinc ions enter the liquid from the rod. As the rod becomes slightly negatively charged as a result, it returns some of the electrons to the zinc ions, resulting in the formation of elemental zinc. There is therefore a constant exchange of ions and electrons at the boundary layer between the rod and the liquid. If you now place a copper rod in the same solution, you will find that some of the zinc ions are deposited on this rod as elementary zinc. This releases electrons from the copper rod so that it becomes slightly positively charged. If you now connect both rods with an electrical conductor, an electric current can be measured between them. In this case, the electrons migrate from the negatively charged zinc electrode to the positively charged copper electrode. Since the electrons now move in the opposite direction compared to electrolysis, in galvanic cells the positive pole is called the cathode and the negative pole is called the anode. Such a structure, consisting of two electrodes with different materials and an electrolyte between them, is called galvanic cells. The electrical potential of the individual electrodes cannot be measured directly, but the potential gradient, i.e. the electrical voltage, between the electrodes can be measured. As current flows between the electrodes, more and more zinc atoms accumulate on the copper electrode. At the same time, more and more zinc ions migrate into the solution, so that the zinc rod slowly dissolves. If the copper electrode is completely covered with zinc, no more zinc ions are available or the zinc rod has completely decomposed, the current flow comes to a standstill, i.e. the battery is empty. There are basically two ways to increase the capacity in this case: On the one hand, you can replace the electrolyte solution with copper sulfate so that more copper ions are available that can accumulate on the copper electrode. Another possibility is the spatial separation of both electrodes in two different vessels. The zinc electrode is in a zinc sulfate solution while the copper electrode is in a copper sulfate solution. In order for a current to flow, ion exchange of the negatively charged ions must be guaranteed. For this purpose, a salt bridge (also called an ion bridge) is used, which in its simplest form consists of a strip of paper soaked in salt water, the ends of which are dipped in one of the two solutions. It is important here that the zinc ions cannot migrate directly into the other vessel, otherwise, the cell will be short-circuited.This page contains 503 words and 2975 characters.
Last modified: 2022-10-01 18:37:41 by mustafa