Surface Tension
Definition
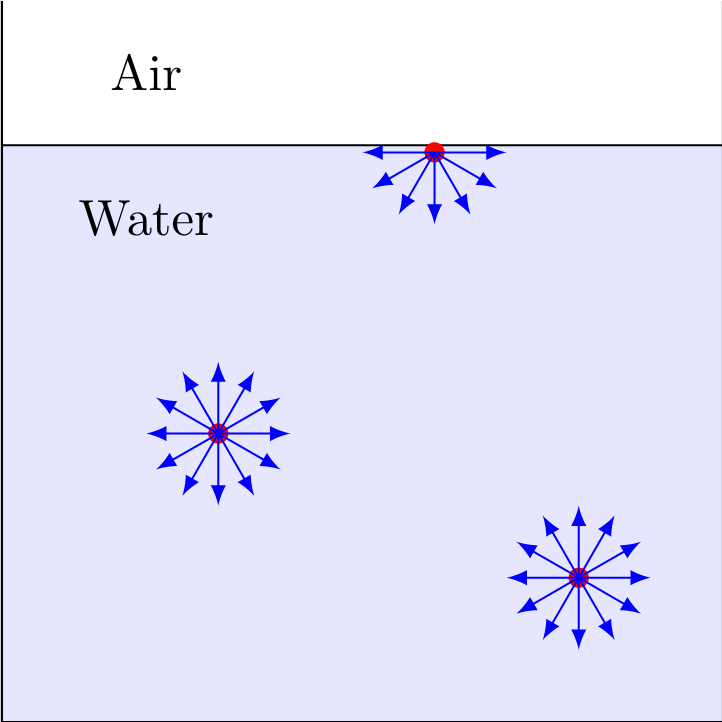
If you now immerse an object, the water must flow around it, which effectively leads to an increase in the water surface along the immersed boundary layer. For this, further molecules have to migrate from the inside to the outside and consequently do work $W$ against the attraction of the particles. One, therefore, defines the fraction of work done divided increase in area $$\varepsilon = \frac{\Delta W}{\Delta A}$$ as so-called specific surface energy with the unit $\mathrm{J}/{m}^2$. Since $\varepsilon$ depends on the liquid used, the specific surface energy must first be determined experimentally. To do this, a wire is bent into a rectangular wire bracket and then a movable cross bracket is attached to it. If the device is immersed in the liquid to be measured, a thin lamella of liquid is formed between the holder and the crossbar. If you now move the stirrup by the distance $\Delta s$, the work $$\Delta W = F\Delta s = \varepsilon 2 L \Delta s = \varepsilon \Delta A$$ has to be performed. With the help of this experiment, the surface energy can be determined. The factor of 2 comes from the fact that both the top and the bottom of the liquid lamella have to be considered. Accordingly, the parameter can be expressed as tensile stress $$\varepsilon = \frac{F}{2L}$$ interpret, which acts tangentially to the water surface. This tensile stress is called surface tension $\sigma$ and has the unit N/m, which is identical to the unit J/m${}^2$ already known.
In everyday life, surface tension plays an important role, for example in the formation of soap bubbles or foam. The surface tension of pure water under normal conditions is around $\sigma = 0.0728\,\mathrm{Nm}$ and would be far too large for the formation of macroscopic bubbles. However, the added soap consists largely of \textit{surfactants}\index{surfactants}, which have a water-soluble (hydrophilic or lipophobic) and a fat-soluble (hydrophobic or lipophilic) part. Surfactants have the task of reducing the surface tension of the water, so that soap bubbles with very large surfaces can also form. The surface tension also ensures that the surface formed wants to make itself as small as possible. This continues until the air pressure in the bubble, which increases as a result, has increased to such an extent that it completely compensates for this effect and creates a soap bubble of constant size.
Experiment